Chain Dimensions
Entanglement & Crossover Length
It is well-known that the viscoelastic properties of many linear polymers vary with molecular weight in basically the same manner. For example, the melt viscosity of short polymer chains (η) is linear proportional to the molecular weight M (or, more precisely, its weight-average, since most polymer samples are not perfectly monodisperse), whereas that of long polymer chains shows a much stronger molecular weight dependence. The smooth crossover between these two regimes occurs at the critical molecular weight Mc whose value is characteristic of a polymer.
η0 = K · M, M < Mc
η0 = K · M3.4, M > Mc
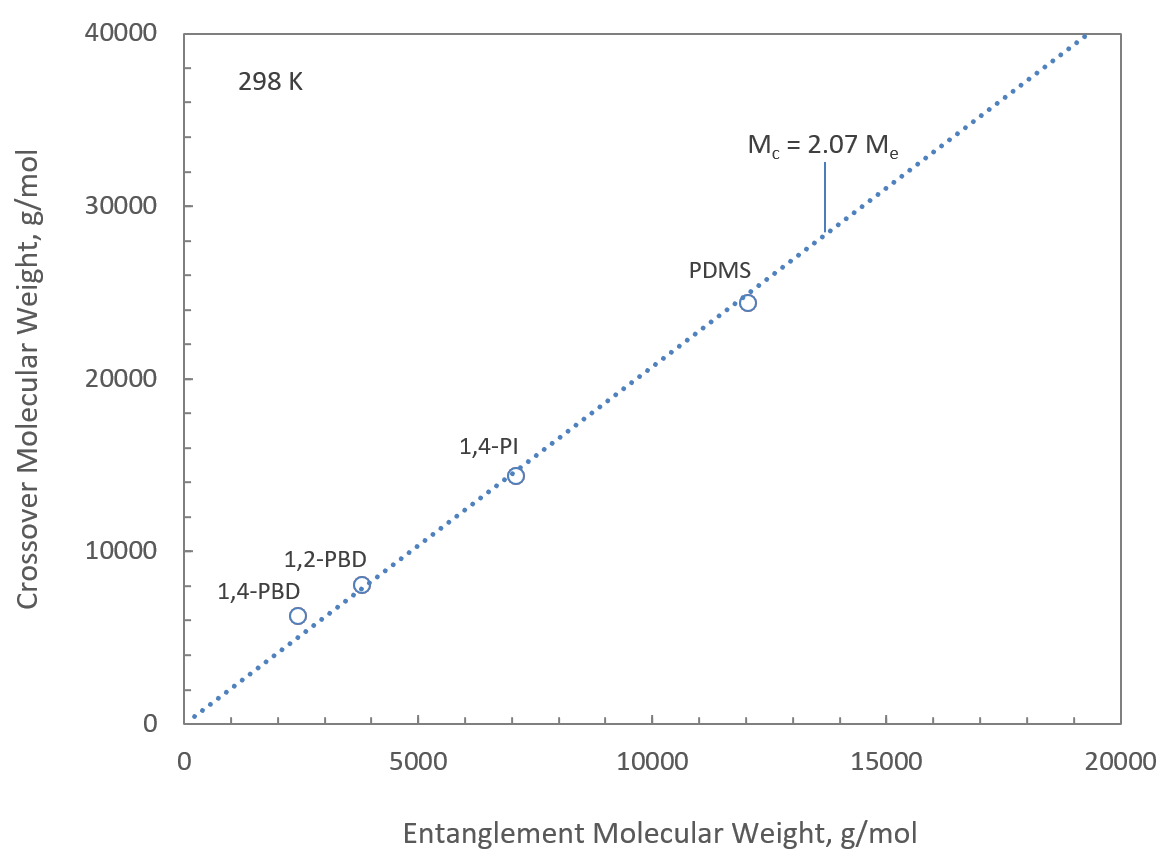
The much stronger dependence of rheology on structure above the critical molecular weight Mc has been explained by entanglement interactions. When each polymer chain is entangled with at least two other chains, the polymers form a three-dimensional network of entanglements.1 Thus, the crossover between the two regimes occurs at about two times the entanglement weight, Mc ≈ 2Me. The parameter Me, is typically calculated from the plateau storage modulus (GN,0) of the polymer in its melt or rubbery state:2
Gp = 4/5 · ρ R T / Me
where R is the universal gas constant (R = 8.314 K-1), T is the temperature, and ρ is the polymer melt density. The relationship Mc ≈ 2Me holds for many linear polymers with a glass transition temperature well below room temperature (see figure below).
However, as Fetter, Lohse, and Milner have shown,3 the crossover molecular weight Mc of many other linear polymers can be noticeably larger or smaller than 2Me, or in other words, the long-held notion that the ratio Mc / 2Me equals about unity for all linear polymers is incorrect. This finding is surprising because of the generally good agreement of many properties in the theta- and melt-state. Krishnamoorti, Graessley et al.4 postulated that most differences in the melt and theta state behavior can be explained by conformational changes induced by solvent molecules, that is, the population of gauche and trans states in solution can differ from that in the melt state depending on the solvent used.
Polymer chain dimensions in a theta-solvent are often evaluated via intrinsic viscosity measurements, as follows:6
Kθ = [η]θ / M1/2 = Φθ ⟨Rh,02⟩ / M3/2
where Kθ is the Mark-Houwink parameter and Rh,0 the hydrodynamic radius of an unperturbed polymer.
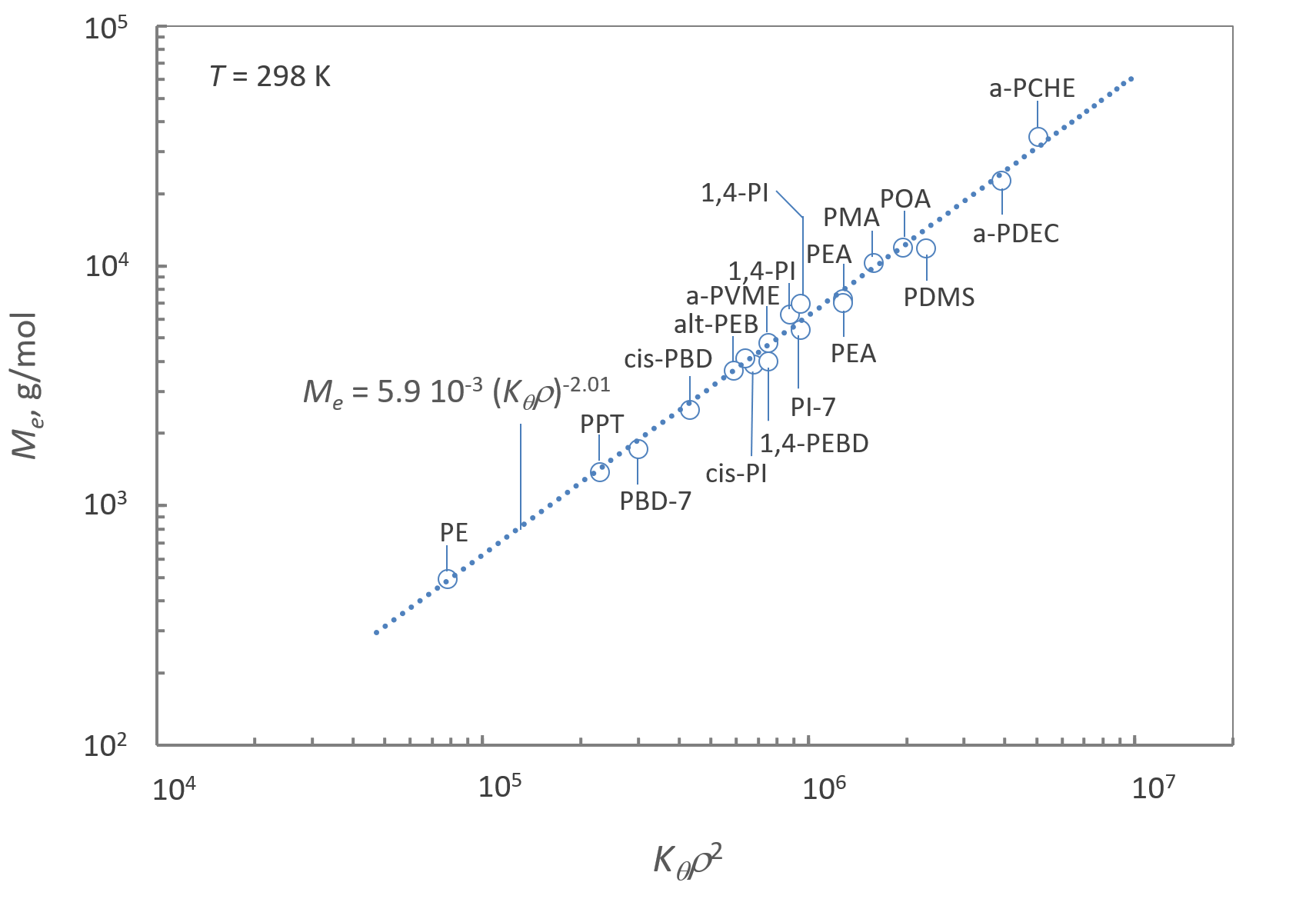
As has been shown by Fetters et al.5, the entanglement weight Me can be calculated from the Mark-Houwink parameter Kθ = [η]θ / M1/2 and the amorphous polymer density ρ:
Me = [Φθnt / NaρKθ]2
where Na denotes the Avogadro number, and nt the number of entanglement strands in a volume with dimensions equal to the diameter of the corresponding reptation tube. Thus, a plot of Me against (ρKθ)-2 should yield a straight line, as it is the case for a large number of polymers (see Figure below).
This result is further proof that the conformational properties of polymers in solution and melt are related to their viscoelastic properties. Therefore, measurements of the molecular dimensions such as size exclusion chromatography, light and small angle neutron scattering, can be utilized to predict the viscoelastic properties of many polymers.
References and Notes
A good analogy is a bowl of spaghetti. When the individual strands of spaghetti are short, they can be easily separated, whereas long strands are very difficult to separate from one the other due to the entanglements.
- M. Doi & S. F. Edwards, The Theory of Polymer Dynamics, Oxford Univ. Press, New York 1986
- Lewis J. Fetters, David J. Lohse, and Scott T. Milner, Macromolecules, 32, 6847-6851 (1999)
- R. Krishnamoorti et al., J. Poly. Sci.: Part B: Poly. Phys., 40, 1768–1776 (2002)
- L.J. Fetters, D.J. Lohse, D. Richter, T.A. Witten, A. Zirkel, Macromolecules, 27, 4639 (1994)